Bring the immune system one step ahead of cancer
Off-the-shelf platform advancing next-generation in vivo immunotherapies to unlock immune response against solid tumors
THEY SUPPORT US
































Redefining immuno-oncology through novel modalities
Mimicking cancer nature
Scalable & cost efficient
Operational excellence
A team of seasoned leaders and innovators in immuno-oncology, backed by €30M in strategic funding.
Our focus: solid tumors
Fast growing needs, insufficient solutions

The challenge
Cancer is a moving, heterogeneous & invisible target
Colorectal cancer (CRC) is the 2nd cause of cancer mortality worldwide,
and is defined by heterogeneity, plasticity and treatment resistances
For these patients: the immune system, natural body’s defenser, is blinded
No immune reaction can be initiated toward tumor cells
Inefficiency of classical immunotherapy
New therapeutic strategies are needed to restore anti-tumor immune response and capture cancer heterogeneity
Our solution:
Next-generation in vivo immunotherapies
From “off-the-shelf” tumor banks to first-in-class immunotherapies
Enriched with the molecular fingerprints of tumor heterogeneity and resistances
Designed to unlock in vivo the immune system to fight back
Inspired by patients, driven by science
Mimicking cancer to make it visible
We model solid tumors complexity using off-the-shelf cell lines, stimulated to reveal cancer-related proteins and validated by proteogenomic and predictive model.
These cells are then tagged with an immune flare (hapten), and inactivated into ghost cells to safely educate the immune system, to target evolving cancer cells and eradicate cancer.
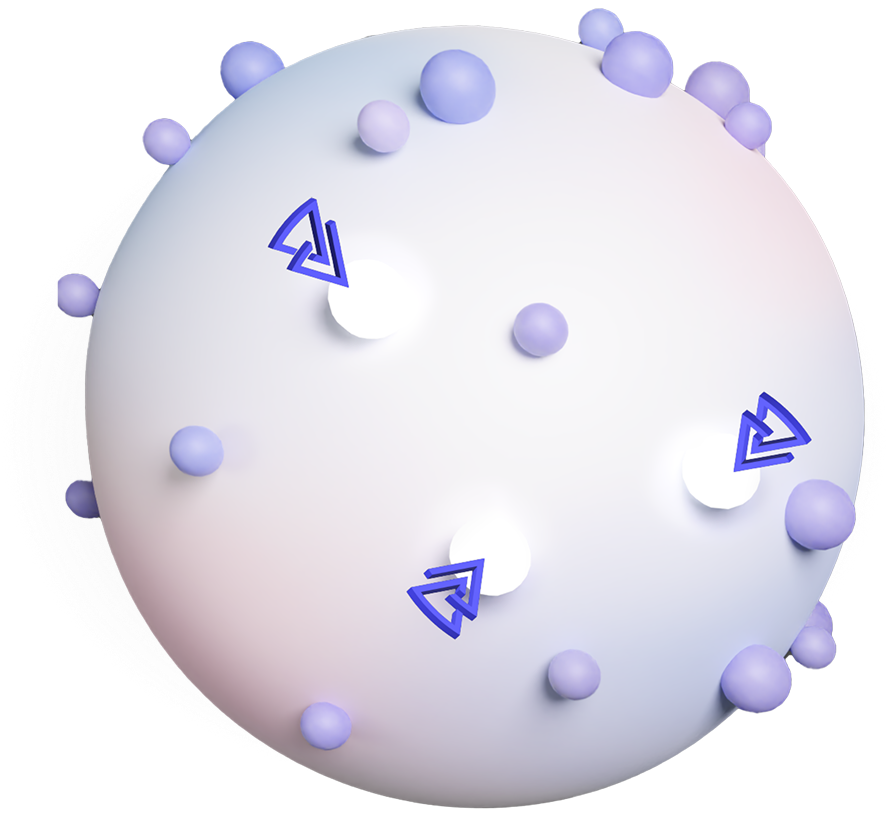
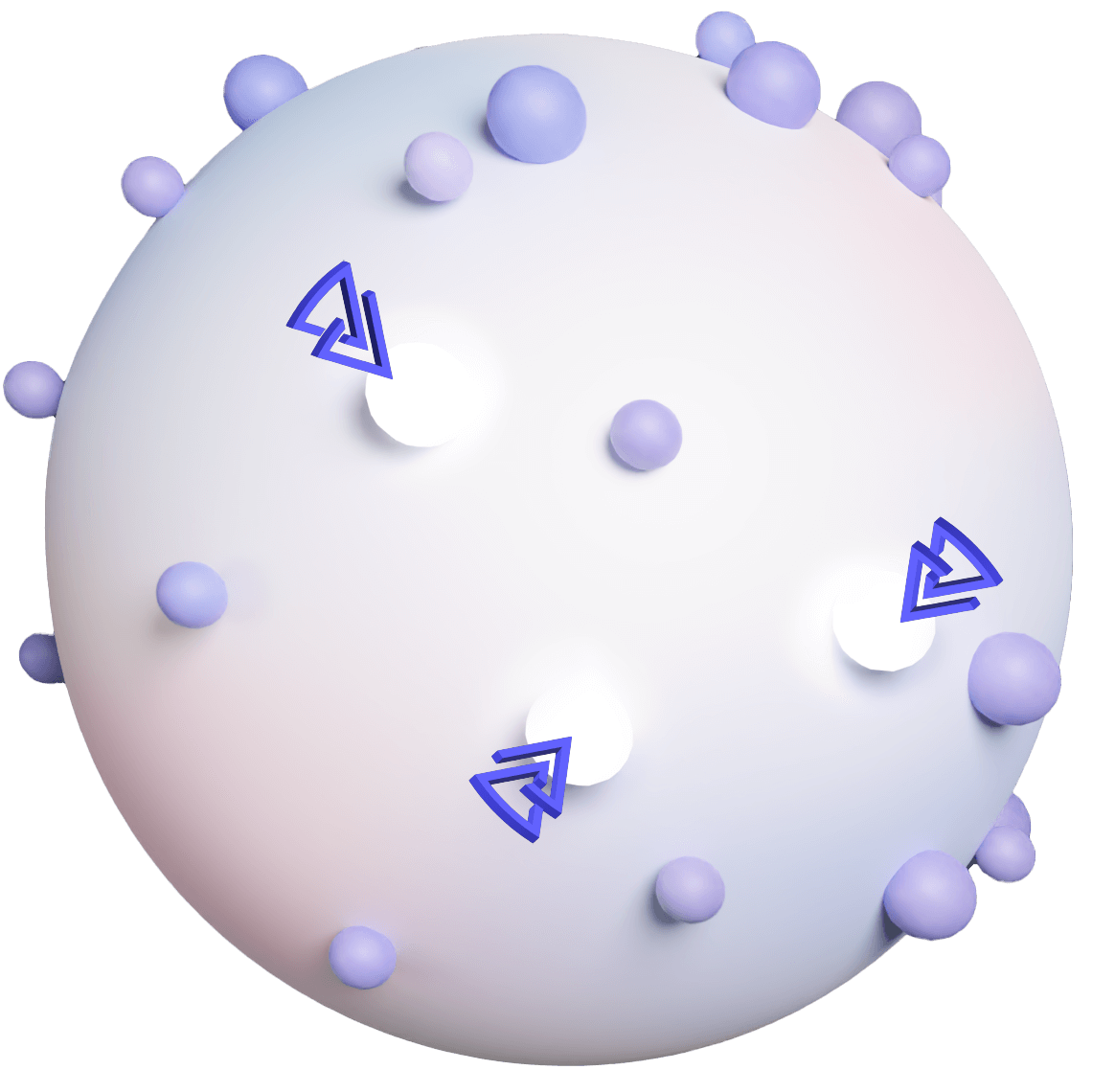
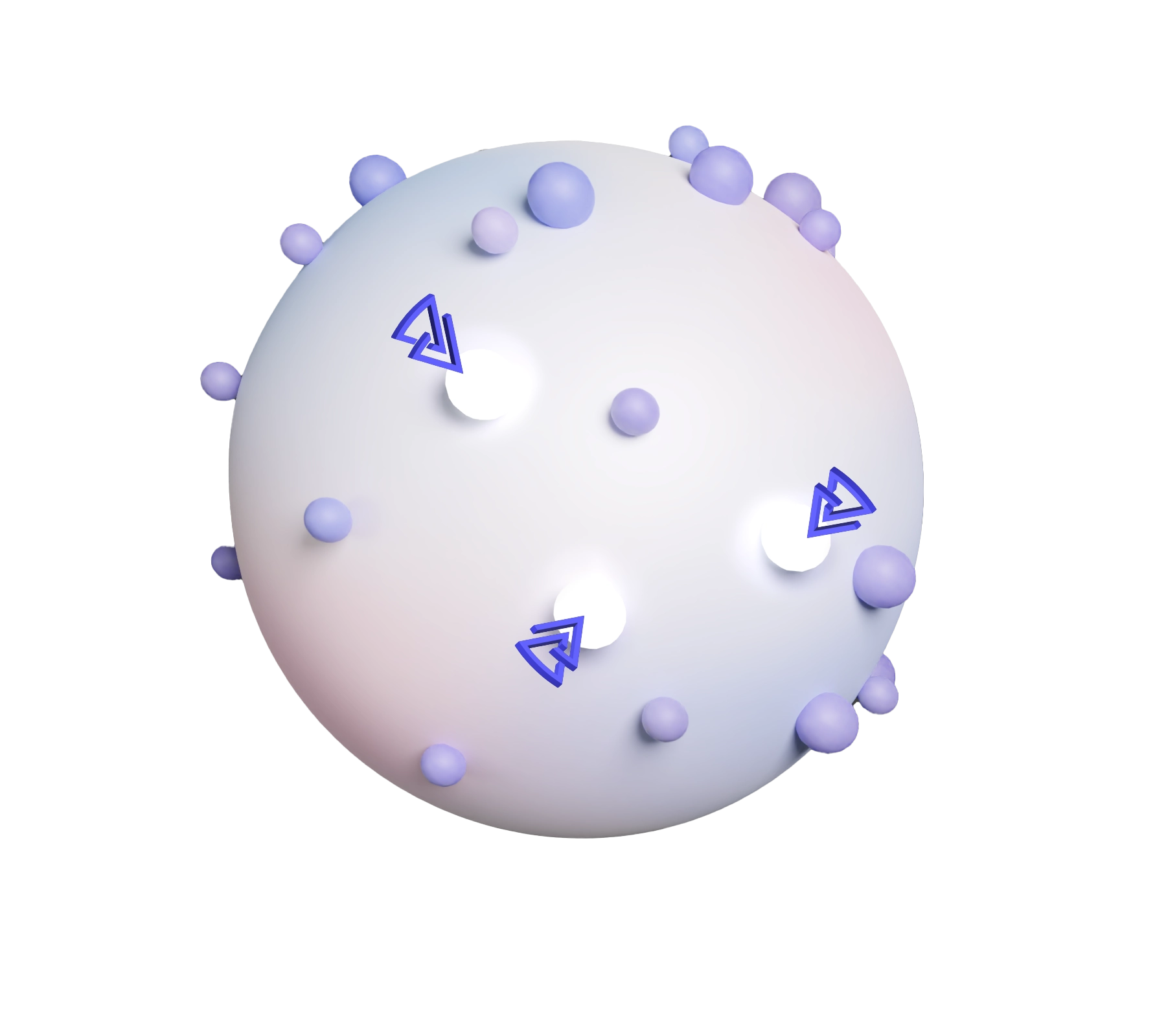
DNFB HAPTEN
DINITROFLUOROBENZENE
CANCER RELATED PROTEINS
From vision to execution
€30M
Invested since 2020
2
Clinical candidates advancing toward IND filing by 2027
9
Clinical sites open in 2025 Phase I/II for colorectal cancer patients in 1L (EU & US expansion planned)
100
patients treatable per batch thanks to our standardized & scalable platform
2 388 000
Eligible patients by 2029
Latest news and research
Brenus Pharma reports favorable tolerability and clinical signals in early preliminary results of first-in-human study for STC-1010 in unresectable metastatic stage MSS CRC patients
Meet Brenus at JP Morgan annual Healthcare conference, San Francisco – 11, 15 Jan 2026
BIO SPACE – Brenus Pharma Raises $25 Million to Accelerate Clinical Trials of Its Precision Anti-cancer immunotherapy














