


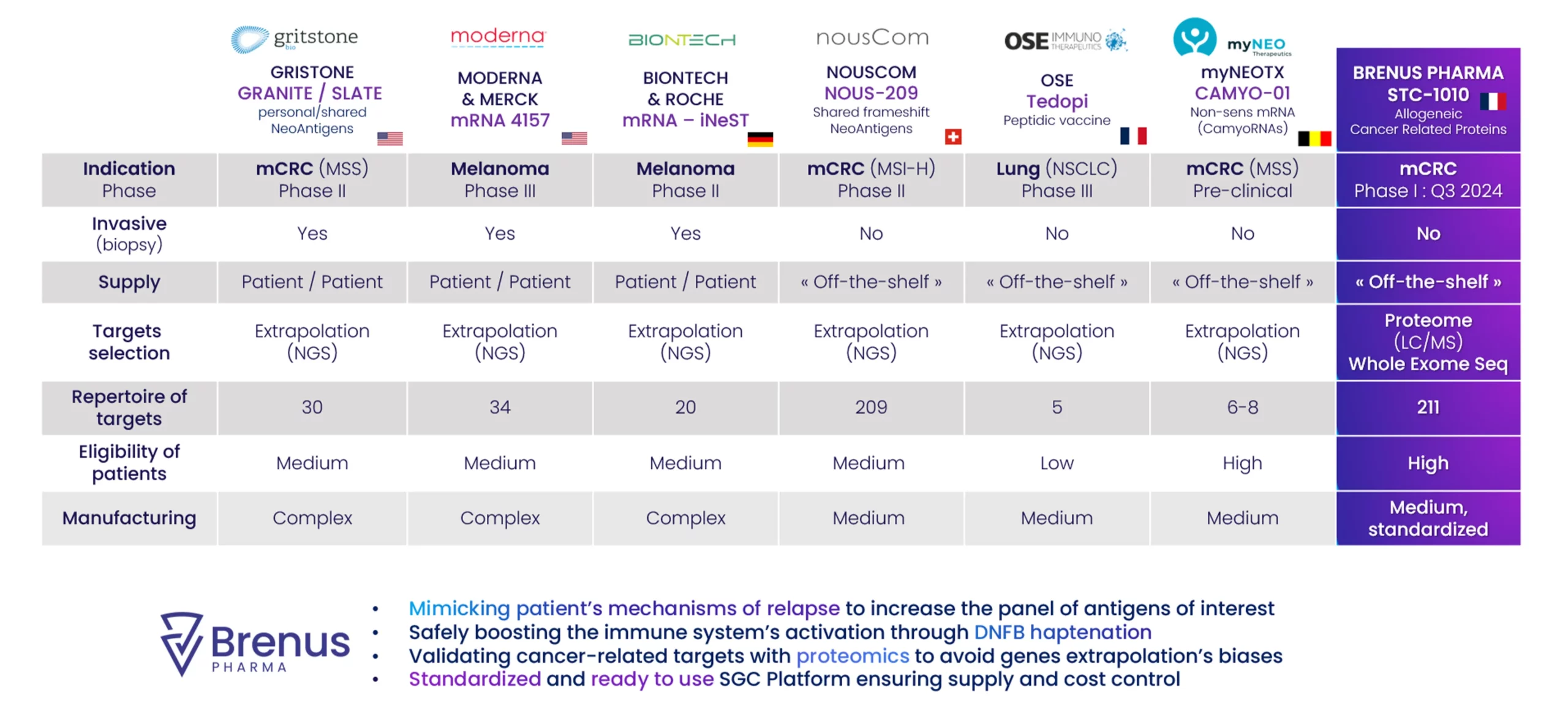
Anticipates risks of tumor escape by targeting over 250 cancer-related antigens driven by relapse mechanism.
Answers huge unmet need in solid tumors : Platform strategy, combining impactful technologies, with standardized steps for a quicker generation of immunotherapy for solid tumors
Preclinical package endoresed with FDA Pre-IND passed.
GMP manufacturing ready.
Top international scientific leader on board & experienced governance in Healthtech industries.
IP by design-platform strategy ; 22 patent granted.
Tailored first-in-class drugs design.
Dynamic immuno-oncology market with mRNA and Personalized Cancer Vaccine.
Ensuring treatment’s availability to a wide population of patient in therapeutic issue.



mCRC preclinical package for the lead candidate, STC-1010, finalized (in-vivo, ex-vivo) endorsed by FDA & FAMHP (EMA). Published in major congress : SITC, AACR, ASCO
Product - GLP, Drugs subsances - GMP Gap Analysis Pre scale-up & Tech transfer - GMP, experienced CDMO
Phase I/II 1L mCRC study synopsis validated (US,BEL,FR)
STC platform design - Granted in Key countries STC-1010 Product - Published
Highly skilled executives and operationals & internationally renowed KOLs on board
Anticipating tumor’s evolution to overpass current limitations
In combination with standard of care
Possible synergy with immunotherapies : They are not necessarily competitors
No other allogeneic vaccine identified to date
Brenus’ manufacturing process overpasses the lack of immunogenicity & educates the immune system with visible and multi-specific targets
Selection criteria of starting material (Allogeneic tumor cell lines)
Brenus’ manufacturing process overpasses the lack of antigenicity & educates the immune system with a broad & higher quality range of tumor antigens