An innovation to mimic patient’s cancer relapsing conditions.
An innovation to mimic patient’s cancer relapsing conditions

Providing a unique value proposal in the field. The successful equation to anticipate disease progression
APC : antigen-presentinf cell, generally dentritic cell
MHC : Major Histocompatibility complex
TCR : T-Cell Receptor
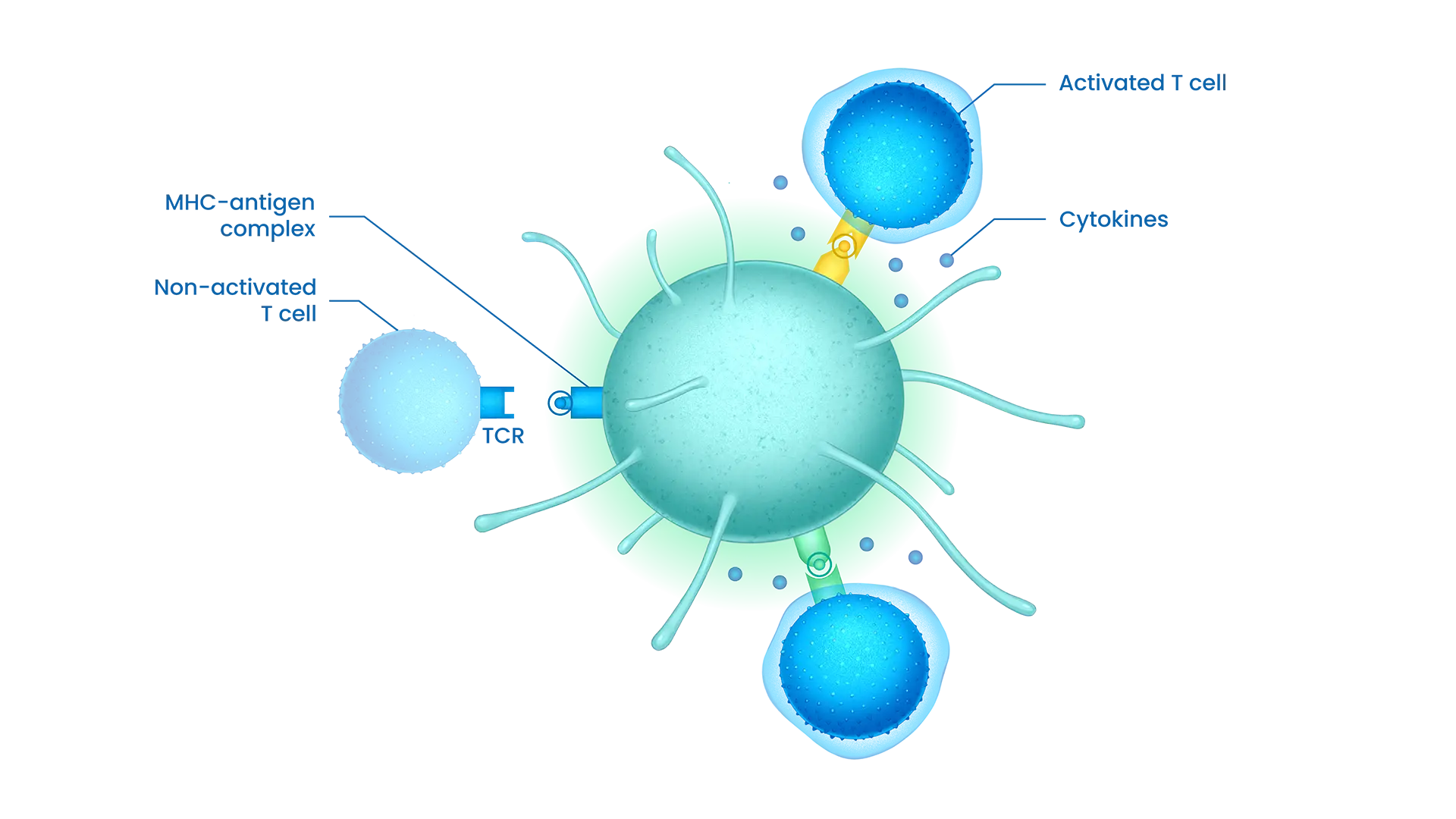
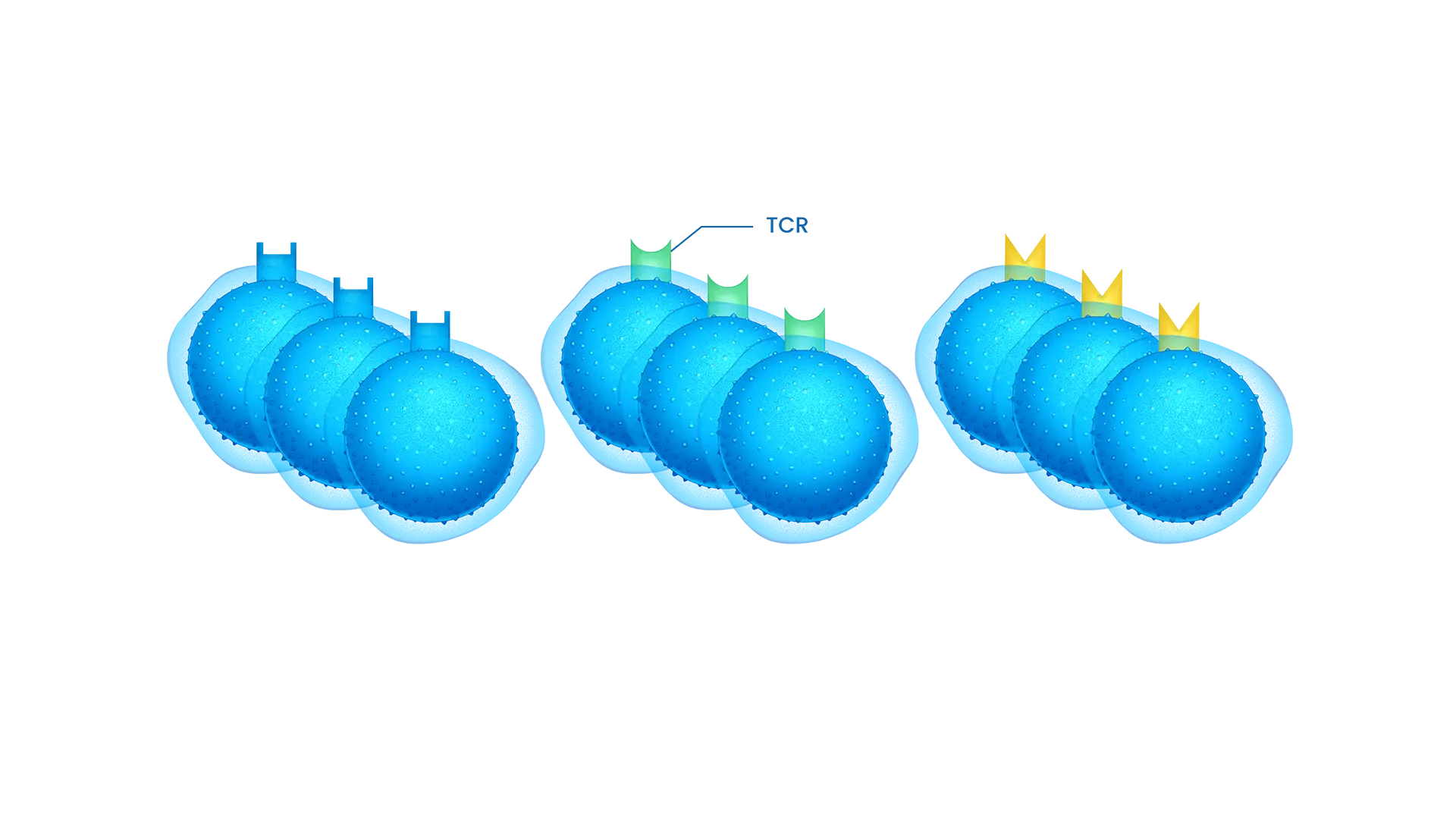
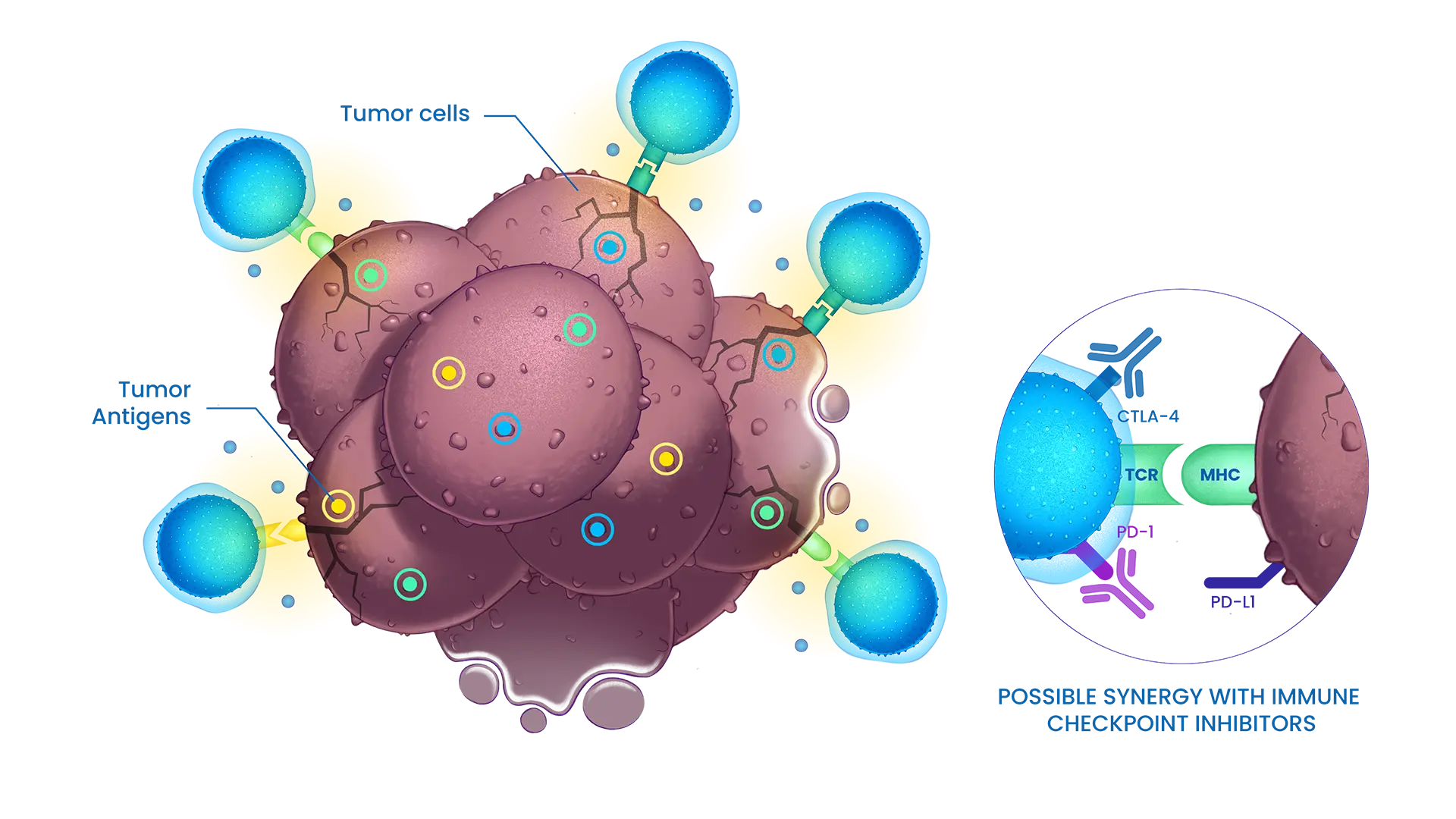
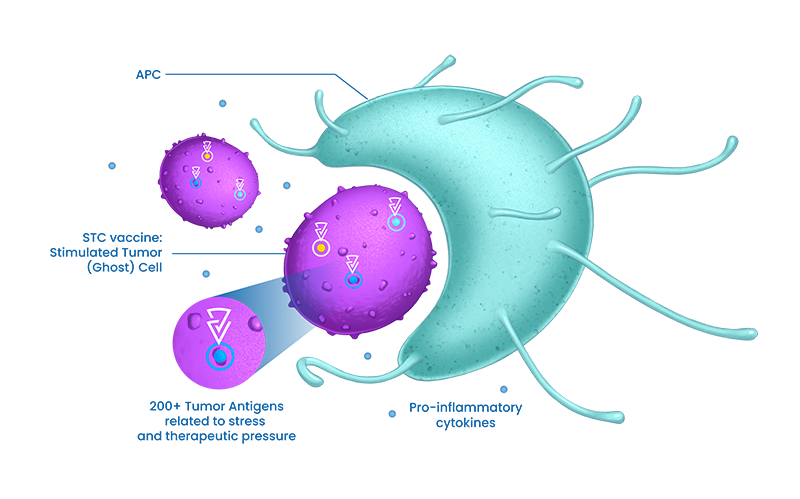
STC platform facilitates the quick generation of new STC treatments to meet patient’s needs in diverse solid tumors indication.
STC – 1010 development: Strong scientific rationale to target other indications
COLORECTAL CANCER
2nd CAUSE OF CANCER MORTALITY WORLDWIDE
OTHER INDICATIONS
STC – 10XX development : Leveraging STC Platform to design first -in-class immuno – oncology products, anticiapting Specific cancers : Ovarian,Lung,Rare cancers…
SOLID TUMOR
STC-1010: our first candidate in metastatic colorectal cancer (mCRC)
STC-1010 targets MSS and MSI-H population.
STC-1010 has a robust and validated preclinical data pack showing:
in colorectal cold and hot mice model
in several model
in colorectal cold and hot mice model
An OFF-THE-SHELF technology allowing control of both time and cost of the manufacturing process.
We are supported by leading figures in clinical immuno-oncology, with 9 early-phase centers committed to the next clinical trial.
“Cancer vaccines continue to show promising clinical results in solid tumors. STC-1010 is a new immunotherapeutic approach based on cancer vaccine mechanism of action for colorectal cancer patients.
In that, “BreAK-CRC” Study is eagerly expected :
CRC is still challenging as current immunotherapies were found only active
in dMMR/MSI-H “hot” CRC.
For the pMMR/MSS population, representing 95% of patients with CRC, there is an important medical need for drugs likely to heat up “cold” tumors and have a real impact for the patient.”
Director of the Early Clinical Unit CLIP2 INCA – CGFL DIJON FR
Director of UMR INSERM 1231 Head of TEAM 1, TIRECs: “Therapies and Immune REsponse
in CancerS” – University of Burgundy DIJON FR

Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua.
See Video here
Brenus’ manufacturing process overpasses the lack of immunogenicity & educates the immune system with visible and multi-specific targets
Selection criteria of starting material (Allogeneic tumor cell lines)
Brenus’ manufacturing process overpasses the lack of antigenicity & educates the immune system with a broad & higher quality range of tumor antigens